Mitochondrial phospholipases
Phospholipases are enzymes that cleave ester linkages in phospholipids and often serve as the rate-determining step in the generation of a wide variety of lipid second messengers. Historically, endogenous phospholipid storage pools in the endoplasmic reticulum and the plasma membrane have been considered the major sources of polyunsaturated fatty acids that are hydrolyzed by intracellular phospholipases during cellular activation. The released polyunsaturated fatty acids serve as substrates for oxidation by a variety of cyclooxygenases, lipoxygenases and cytochrome P450s to generate a rich repertoire of lipid second messengers. However, recent studies using a wide variety of genetic, pharmacologic and mass-spectrometric approaches have demonstrated a prominent role for the activation of mitochondrial phospholipases and the hydrolysis of mitochondrial phospholipids in the production of a diverse array of signaling molecules by multiple distinct mechanisms.
Mitochondria fulfill multiple cellular functions regulating cellular metabolism, bioenergetics, signal transduction and cell fate. These pleiotropic roles of mitochondria are integrated precisely to promote metabolic efficiency and bioenergetic flexibility, which allows each cell to fulfill its physiologic functions and adapt to external perturbations.
Recent studies have demonstrated the prominent roles of lipid second-messengers and mitochondrial reactive oxygen species, or ROS, as physiologic signaling moieties. Conversely, the excessive and maladaptive production of ROS in disease states leads to the generation of toxic chemical species that promote mitochondrial dysfunction and cell death (e.g., necrosis through opening of the mitochondrial permeability transition pore, or mPTP, or apoptosis through the release of cytochrome c).
As such, strategies that modulate the activities of mitochondrial phospholipases and their production of downstream lipid second messengers offer a fertile area for pharmacologic intervention to attenuate the progression of disease processes.
Years ago, we in murine myocardial mitochondria, iPLA2γ (also known as PNPLA8) and by cloning the gene encoding the protein, identified a mitochondrial localization sequence at the N-terminus. To assess the roles of iPLA2γ in cellular function, we generated iPLA2γ knockout mice through of the iPLA2γ active site, thereby eliminating iPLA2γ enzymatic activity as well as detectable protein. Moreover, genetic deletion of iPLA2γ altered cardiolipin content and molecular species distribution that was accompanied by defects in mitochondrial function.
Subsequent studies demonstrated a marked decrease in oxidized lipid second-messenger production in iPLA2γ knockout mice in multiple tissues to a variety of . Intriguingly, human recombinant iPLA2γ demonstrated a remarkable regiospecificity hydrolyzing phospholipids containing polyunsaturated fatty acids (e.g., arachidonic and docosahexaenoic acids) at the sn1 position to generate 2-arachidonoyl lysolipids with the concomitant release of potentially toxic saturated fatty acids in the inner . We also demonstrated that iPLA2γ readily catalyzed the hydrolysis of plasmalogens containing arachidonic acid at the sn2 position resulting in the direct release of arachidonic acid and the production of lysoplasmenylcholine.
Collectively, these results demonstrated that iPLA2γ contributes to release of polyunsaturated fatty acids through both the traditional direct release of arachidonic acid (orange line in figure) as well as through a sequential two-step process initiated by sn1 hydrolysis generating 2-AA-LPC and the subsequent release of arachidonic acid by cellular lysophospholipases (blue lines in figure).
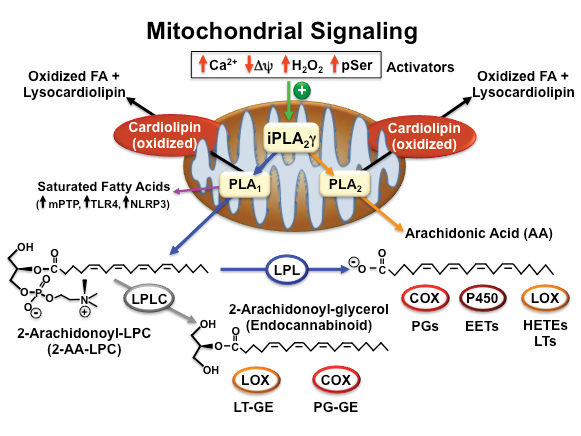 Generation of lipid second messengers by iPLA2γ and downstream enzymes
Generation of lipid second messengers by iPLA2γ and downstream enzymes
Although iPLA2γ does not require divalent cations for catalytic activity, it is activated markedly by physiologic increments of calcium ion present in the mitochondrial matrix during . Accordingly, we compared the generation of calcium-stimulated eicosanoid lipid second messenger in mitochondria from wild-type mice to mitochondria isolated from iPLA2γ knockout mice. The results demonstrated a dramatic decrease in calcium-stimulated eicosanoid production in mitochondria isolated from iPLA2γ .
In previous studies, Douglas R. Pfeiffer and co-workers demonstrated that a mitochondrial calcium-independent phospholipase modulated the opening of the mPTP and the release of . However, the molecular identity of the responsible enzyme was unknown. Accordingly, we used the iPLA2γ knockout mouse to demonstrate that genetic ablation of iPLA2γ markedly attenuated the calcium-induced . Collectively, these studies demonstrate the importance of mitochondrial iPLA2γ in the generation of lipid second messengers, cellular signaling and cell-fate decisions.
The mechanisms underlying the activation of iPLA2γ are an area of active investigation. Through genetic and pharmacologic approaches, we demonstrated that physiological increases in mitochondrial matrix calcium activate . Recently, studies by Pfeiffer and co-workers demonstrated that decreases in mitochondrial membrane potential activate . In addition, Petr Jezek and co-workers demonstrated that iPLA2γ is activated by . Importantly, complement treatment of glomerular epithelial cells resulted in stimulation of iPLA2γ by serine phosphorylation catalyzed by . Based on these studies, it is apparent that iPLA2γ serves as a prominent mediator of cellular bioenergetic and lipid signaling in multiple cell types in a spatial and context-dependent manner.
Valerian E. Kagan and co-workers, by identifying the iPLA2γ-mediated release of oxidized polyunsaturated aliphatic chains in cardiolipin after cellular stress, elegantly demonstrated the importance of mitochondria in the production of signaling metabolites in response to . When cardiolipin binds to cytochrome c, a conformational change in cytochrome c occurs, transforming cytochrome c from an electron carrier to a potent peroxidase with a remarkable specificity for oxidation of aliphatic . Using a powerful combination of mass-spectrometric technologies, genetic approaches and pharmacologic inhibition, they demonstrated that cytochrome c-oxidized cardiolipin aliphatic chains are released in response to cellular stress by an (R)-BEL inhibitable . These results demonstrate a novel mechanism for lipid second-messenger generation where polyunsaturated chains on cardiolipin are first oxidized by cytochrome c and subsequently hydrolyzed by iPLA2γ to serve as lipid second messengers after hydrolysis, leading to the direct release of a panoply of known, and as yet incompletely characterized, signaling molecules.
Collectively, these studies identify the importance of mitochondrial phospholipases in serving critical roles in cellular signaling, bioenergetics and cell-fate decisions through the generation of a diverse array of lipid second messengers. Through these mechanistic insights, numerous therapeutic opportunities for the treatment of mitochondrial-mediated disease states have been identified.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

ApoA1 reduce atherosclerotic plaques via cell death pathway
Researchers show that ApoA1, a key HDL protein, helps reduce plaque and necrotic core formation in atherosclerosis by modulating Bim-driven macrophage death. The findings reveal new insights into how ApoA1 protects against heart disease.

Omega-3 lowers inflammation, blood pressure in obese adults
A randomized study shows omega-3 supplements reduce proinflammatory chemokines and lower blood pressure in obese adults, furthering the understanding of how to modulate cardiovascular disease risk.

AI unlocks the hidden grammar of gene regulation
Using fruit flies and artificial intelligence, Julia Zeitlinger’s lab is decoding genome patterns — revealing how transcription factors and nucleosomes control gene expression, pushing biology toward faster, more precise discoveries.

Zebrafish model links low omega-3s to eye abnormalities
Researchers at the University of Colorado Anschutz developed a zebrafish model to show that low maternal docosahexaenoic acid can disrupt embryo eye development and immune gene expression, offering a tool to study nutrition in neurodevelopment.

Top reviewers at ASBMB journals
Editors recognize the heavy-lifters and rising stars during Peer Review Week.

Teaching AI to listen
A computational medicine graduate student reflects on building natural language processing tools that extract meaning from messy clinical notes — transforming how we identify genetic risk while redefining what it means to listen in science.

